What is electrochemical CO₂ reduction?
It is a key technology for achieving carbon peak and neutrality goals, using renewable electricity to convert CO₂ into high-value fuels and chemicals.
However, conventional gas diffusion electrodes suffer from unstable three-phase interfaces, declining product selectivity at high current densities, and performance degradation due to electrolyte acidification. For instance, commercial carbon paper electrodes remain stable for only a few hours, severely limiting scalability.
Recently,the team led by Assistant Professor Zhang Xiaolong (Faculty of Materials Science and Energy Engineering, Shenzhen University of Advanced Technology)published a paper titled“Ag-based integrated gas diffusion electrode with enhanced three-phase interface for electrochemical CO₂ reduction” inApplied Catalysis B: Environment and Energy.
Screenshot of the published article
Addressing core industrialization challenges, the team developed a high-performance integrated gas diffusion electrode, providing a new route toward efficient and stable artificial carbon cycling systems.
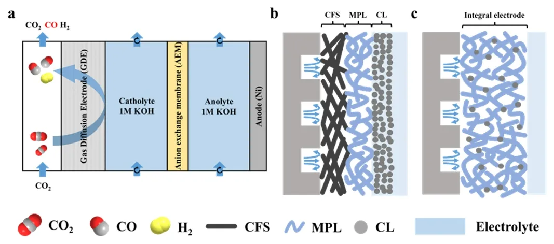
Schematic of conventional layered structure vs. integrated gas diffusion electrode in a flow cell
The team proposed a“structure–function integration” design strategy, constructing a 3D hydrophobic conductive network with PTFE and conductive carbon black, then loading ultrasmall 3 nm Ag nanoparticles via mixed-solvent impregnation to form a novel integrated gas diffusion electrode (Ag-IGDL).
The electrode offers three key advantages:
1
1. A 3D hydrophobic conductive network dramatically enhances stability: The expanded three-phase interface maintains CO Faradaic efficiency (FE_CO) >95% across 100–600 mA cm⁻², with 20-fold higher stability than conventional layered electrodes and continuous operation exceeding 60 hours.
2
2. Ultrasmall Ag nanoparticles boost catalytic activity: By tuning the water/isopropanol ratio (1:1), uniform 3D distribution of Ag nanoparticles exposes more active sites, achieving a mass activity of 7.04 A mg⁻¹ (FECO >90%)—far surpassing existing Ag-based catalysts.
3
3. Optimized interface suppresses side reactions:Moderate pore size (0.13 μm) and hydrophobicity (contact angle 139°) balance CO₂ mass transport and retention, minimizing non-productive CO₂ absorption in alkaline electrolyte and suppressing the hydrogen evolution reaction (HER).
Thisstudy achieves a critical leap from lab to industry inwide-range efficiency, long-term stability, and mechanistic innovation. For example, FE_CO remains >95% across 100–600 mA cm⁻², overcoming selectivity decay at high current densities; at 200 mA cm⁻² in an MEA electrolyzer, the electrode operates stably for >60 h with FE_CO decay <10%, while conventional electrodes fail within 3 h. Humidity tests and active-site validation confirm that Ag nanoparticles throughout the 3D network participate in catalysis, revealing synergistic optimization of mass and electron transport by the integrated structure.
This breakthrough not only establishes a new paradigm for high-efficiency CO₂ reduction electrodes but also demonstrates Shenzhen University of Advanced Technology’s original R&D strength in energy materials and devices. The integrated electrode strategy is extensible to other metal-based catalysts and is expected to propel electrochemical carbon cycling from lab to industry, providing crucial technological support for China’s carbon peak and neutrality goals.
Corresponding affiliations: Shenzhen University of Advanced Technology and Shenzhen Institutes of Advanced Technology (SIAT), Chinese Academy of Sciences. Dong Yingjun is the first author, and Zhang Xiaolong is the corresponding author click “Read more” at the end of the article for paper details.